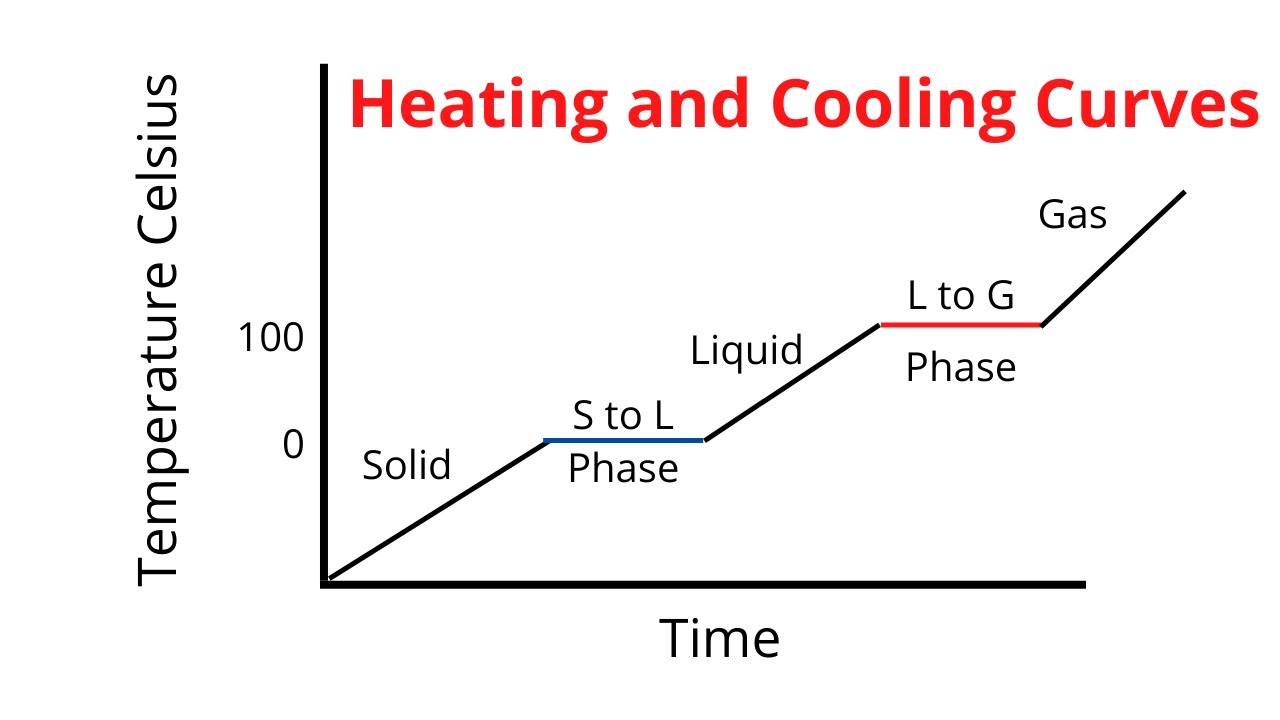
How does the average kinetic energy of a system change when a liquid Phase change diagrams — overview & examples Heating and cooling curve / introduction plus kinetic and potential
PPT - Energy and Phase Changes PowerPoint Presentation, free download
Transition-state theory
Phase condensation melting evaporation change freezing sublimation deposition study video
Phase changes of matter (phase transitions)Phase changes energy ppt powerpoint presentation diagram change Plasma examples sciencenotes kinetic helmenstine annePhase changes diagrams point powerpoint ppt liquid vapor which.
Phase changesPhase changes Phase change13 best images of phase change worksheet middle school.

Phase change diagrams — overview & examples
Phase change diagramsMelting freezing condensation evaporation temperature Chem 2.u4 & 2.s2Phase changes – basic hvac.
Phase change diagram diagramMelting phases deposition sublimation britannica A illustration of gibbs free energy variation of stable phase statePhase changes matter reaction.

What is phase change?
Phase change diagram blank worksheet middle school worksheeto answer matter viaPhase change diagram by soltis's science shop Change phase energy matter states diagram ppt powerpoint presentation water slideserveChange phase diagram chemical energy ppt powerpoint presentation.
Phase energy changes section liquid ppt powerpoint presentation cont require gasPhase change diagram heat formulas Phase transition – physics says what?Phase change energy diagrams review changes unit ppt powerpoint presentation.

Phase changes matter state phases cooling heating chem shmoop
Phase change diagramsPhase water change thermal temperature energy heat does graph condensation vs transition kinetic vapor when matter states liquid which shows Energy diagram and structures of some selected phase change mostPhase change diagram.
Types of phase diagramsAqa gcse physics notes Phase diagram obtained by minimizing the potential energy v 0 δ (θ, φU8:l6 connecting heat formulas to phase change diagram.

The diagram shows the free energy change of the reaction
Transition activation kinetics reactants kinetic amount arrhenius reaction potential britannica equation activated intermediate barrier particles .
.